Rock of the Month December 2022
Apophyllite
This rock of the month is a little different from previous months. “Apophyllite” is a common mineral that many collectors often have multiple samples of in their collection. It is seen in booths all over mineral, gem, and rock shows from multiple locations, but the name apophyllite is not representative of one mineral but a group of minerals known as the apophyllite group. Apophyllite was used to refer to one specific mineral before 1978 and is now used to refer to the mineral group.
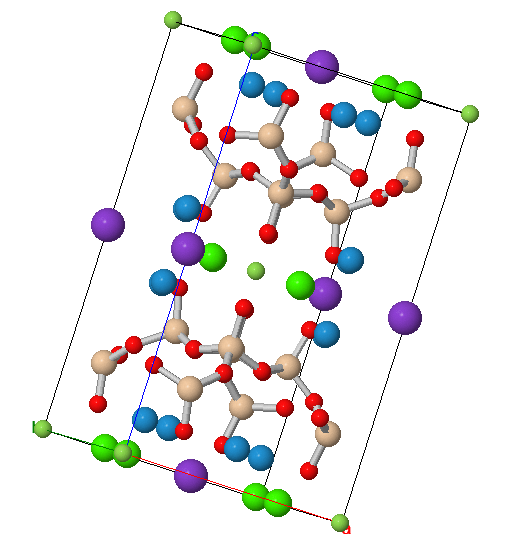
The apophyllite mineral group is a set of phyllosilicates that create a series made of the following members: fluorapophyllite-(K) KCa4Si8O20(F,OH)·8H2O , hydroxyapophyllite-(K) KCa4Si8O20(OH,F)·8H2O, fluorapophyllite-(Na) NaCa4Si8O20F·8H2O, fluorapophyllite-(Cs) CsCa4(Si8O20)F·8H2O, fluorapophyllite-(NH4) NH4Ca4(Si8O20)F⋅8H2O, and hydroxymcglassonite-(K) KSr4Si8O20(OH)·8H2O. Members of this group share a layered sheet structure created by a silicon-oxygen tetrahedra. The tetrahedra create a center structure with 4 fold symmetry yielding 8 member rings creating the sheets. Two of these sheets create the outline for the crystal structure for the series. In between these sheets is where the members differ from each other. In the most common member of the series fluorapophyllite-(K) potassium ions interact with the free oxygen and water around the rings. This interaction points the majority of the structure along the c+ and c- minus axis of the crystal. We will now focus on the most common series member fluorapophyllite-(K).
Figure 1. An angled view of the structure of fluorapophyllite-(K)
Two molecules of fluorapophyllite-(K) are needed to create a unit of the crystal. This structure is the base of the crystal lattices that make up the visible crystal we collect. In the figures above the two rings can be seen creating the central sheet structure. Water, potassium, and fluorine can be seen in between the ring structures, leading to a perfect cleavage plain at {001} across the x axis of the crystal. This leads to many samples in the ditetragonal prism crystal class that form dipyramid and prism combinations losing the points of their crystals. Fluorapophyllite-(K) can also form a pseudo cubic crystal shape so not all blocky crystals have been damaged. One of the least common forms of fluorapophyllite-(K) is bladed where it can form thin bladed clusters and rosette-like structures.
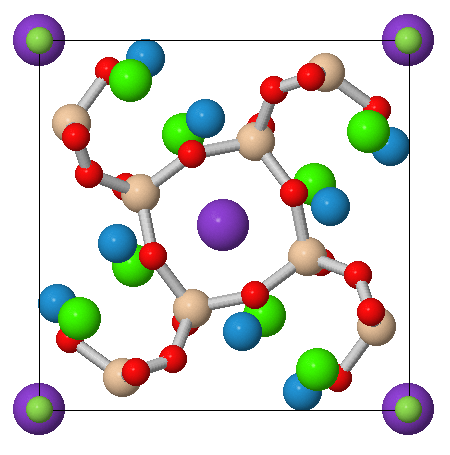
Figure 2. A view of
fluorapophyllite-(K) along
the C axis of the crystal
To most collectors the most desirable member of the apophyllite family are bright green to teal toned crystals of Fluorapophyllite-(K) from India. Many of these samples are produced in Maharashtra State, Jalgaon district from multiple quarries and mines. The green tones in the crystal are caused by vanadium inclusion. Samples can be found as clusters, individual crystals on matrix, as floaters, or as combination pieces (commonly found with a variety of zeolites). The most desirable crystal exhibits colors bordering on neon with a glass like levels of clarity and high luster with varying sizes. When combined with aesthetic positioning and other quality zeolites, pieces can create jaw dropping displays and reach thousands in price.
The name apophyllite comes from two Greek words first ἀπό (apo) meaning away from and second φύλλον (phyllos) meaning leaf. The name comes from what happens when apophyllite is heated. The water in between the two rings breaks loose causing the crystals to begin to flake off in small sheets, making the Greek name quite fitting.
The apophyllite series is a common set of minerals appearing all around the world. Members have been found at nearly a thousand locations listed on mindat.org. The mineral can be found across the United states, Europe, Russia, Africa, and Japan. The most famous locations for the series are in India, however an honorable mention are the incredibly rare pink apophyllites found in Harz Mountains of Germany. Finding one of these samples is incredibly difficult and costly. These samples are different from the more common iron included red apophyllites.
The apophyllite series is often found in igneous and metamorphic basalts. Inside of these rocks, bubbles often form, which can act as a catch for water containing all of the required materials for the crystal to form. Zeolites and apophyllite form at low temperatures and pressures making them common and easily accessible, great news for most collectors.